Quantum Dots
Recent years, we have heard frequently the term of ”quantum dots”. But, do we really know what, exactly, are quantum dots (QDs)? In this article, we would like to give you a short introduction about quantum dots, their unique properties and also their applications. QDs are the core technology that is developed in Quantum Solutions.
Quantum dots technology currently is in uptrend and have been used in the last years to improve color quality in displays and lighting. Even though QDs were discovered firstly in 1980, it took quite sometime until they finally started to be utilized in the real applications and brought from research/lab to the market. QDs were firstly introduced in 2013 in TV display by SONY. Since then, significant innovative discovery in quantum dot technology has enabled further development and the importance of quantum dots in the today’s world cannot be over-emphasized. They serve multiple purposes, especially in the areas of optoelectronic devices, energy, biological and chemical applications. With more study into quantum dots, who knows how much potential will be discovered?

What are Quantum Dots?
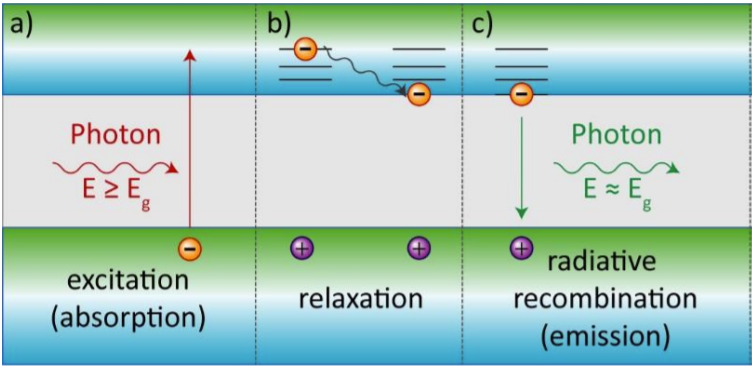
Quantum dots are basically nanocrystals of semiconductor materials. The term “dots” refers to their tiny size which is typically in the nanoscale range of 2-10 nm (nanometer), and the “quantum” term refers to their physical properties that can show “quantum confinement effect”. Quantum confinement effect only happens on nanoscale size and it does not appear in bulk semiconductor material. This effect enables QDs unique behavior under the light (in other words “photons”) exposure. In a semiconductor material, incident photon can promote an electron from the valence band (VB) to jump to the conduction band (CB) creating “electron-hole” pair. This is called excitation process. But not all the photons can make it happen, but only those whose energy is equal to or larger than QDs bandgap (Eg) – the energy difference between valence and conduction bands energy levels. Electrons that are excited into the CB can lower their energy to a state near the band edge by dissipating their excess energy mainly as heat. The process called “relaxation process”. Then the magic starts – the excited electron can recombine with the hole, releasing its gained energy as a new photon. As this photon emits from the band edge of the conduction band to the band edge of the valence band, it emits the energy of the bandgap. This process called ‘radiative recombination’, and it is the main form of photoluminescence (PL) in semiconductors (Figure 2). Our human eye can see these emitted photons in the form of the light. Depending on the energy of the bandgap, we can see various colors: blue, green, red, yellow (Figure 1) or even invisible for us UV and near infrared colors.
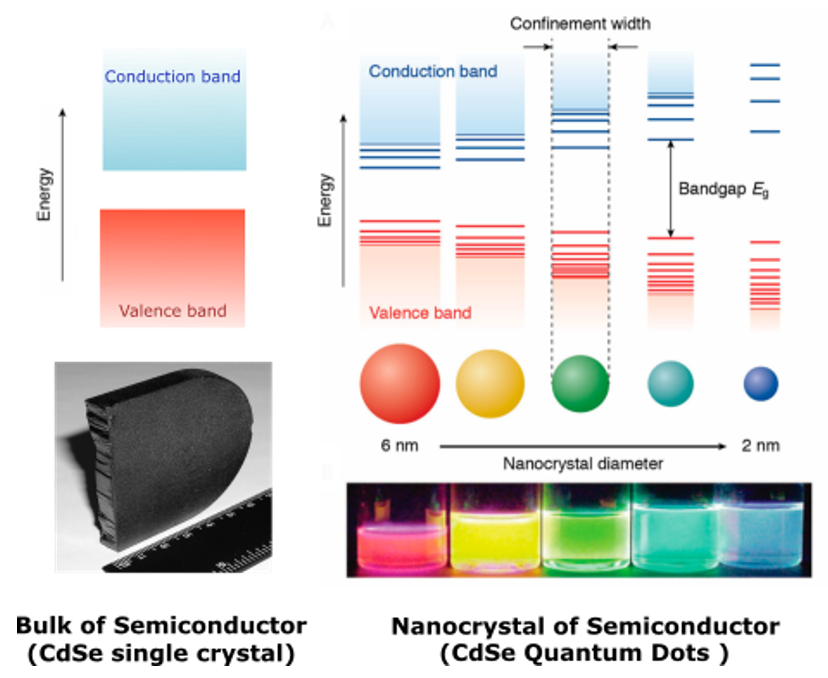
In quantum dots the color of emitted light is determined by the energy of the bandgap. The unique feature of the quantum dots that it is possible to tune the bandgap for THE SAME MATERIAL by simply changing the quantum dot size. Let’s take an example of Cadmium Selenide (CdSe) as a semiconductor material. CdSe bulk (single crystal) does not have quantum confinement effect and doesn’t emit the light under excitation by photons. (Figure 3). But if the size of this crystal is decreased, after reaching of the certain size (usually below 50 nm) quantum confinement effect takes place and these nanoparticles start to emit the light. As QDs becoming more and more smaller, confinement begins to affect the exciton wave function, inducing changes in the density of electronic states and in the energy level separation, which are manifested in an increase of the bandgap with decreasing size and the appearance of discrete energy levels near the band edges. As a result, the optoelectronic properties of QDs become strongly size dependent, making it possible to tune the photoluminescence (PL) of QDs through a wide spectral window by controlling the size of the QDs. For example CdSe QDs with the size around 6 nm emit red light (corresponding Eg is 2.0 eV and emitted light wavelength 630 nm), with the size around 3 nm emit green light (corresponding Eg is 2.3 eV and emitted light wavelength 550 nm), with the size around 2 nm emit blue light (corresponding Eg is 2.7 eV and emitted light wavelength 460 nm) (Figure 3).
Different example of Quantum dots
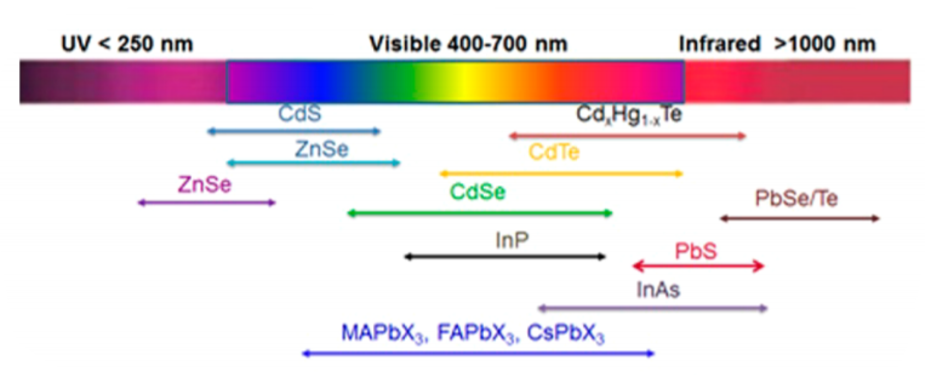
In order to control and modify a particle to the point that it can absorb or emit specific colors of light, the size of the semi-conductor can be adjusted. But not only the size, the properties of a quantum dot also depend to its shape, structure, and composition. For instance, a quantum dot can be spherical shape, cube shape, solid or hollow, and this state will of course affect its properties as well. Also, the properties can be controlled by their composition as well. For example, different type QDs such as CdSe, CdS, ZnSe, InP, ABX3 Perovskites, InAs, PbS will give different emission spectrums range (UV, visible or near infrared) and their size/shape can be controlled also to adjust the spectrum. (Figure 4)
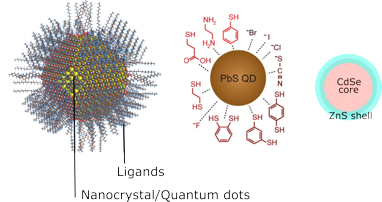
Typical structure of Quantum Dots
As structural, quantum dots consist of the semiconductor nanocrystal itself on which is covered by surface ligands. For example, PbS QDs, it has PbS nanocrystals and may be protected by ligands with different functional group such as thiol, carboxylic acid etc. (as shown in Figure 5). Ligands are typically organic molecules but sometime inorganic ions can be used as well. The ligands have role in providing QDs colloidal stability and dispersibility. Ligands also affect the QDs morphology and function.
The nanocrystals may consist also core-shell structure such as in CdSe or InP-based QDs. The shell help on passivation the surface defects that typically happen in these QDs. The presence of shelling will reduce the surface trap state so that will improve the photoluminescence of QDs.
Applications
Quantum dots can be applied in a wide range of applications, including—but not limited to—optoelectronic devices, imaging, horticulture, information storage, catalysis, solar cells and biomedical imaging. (Figure 7)
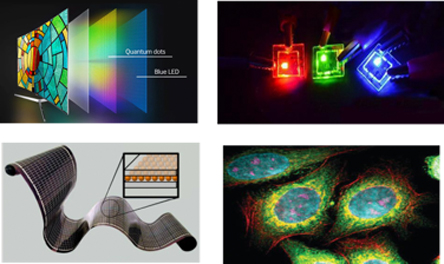
Quantum Dots show promise in image sensors for capturing either short wave or near infrared light or high energy X-ray or UV light. For example, quantum dots can be assembled with silicon CMOS sensor to detect visible and near infrared light in the same pixel. Such near infrared sensing is getting very important in such devices as photo-cameras (for biometrics), autonomous cars (obstacles detection), machine vision (quality control and product inspection), AR and VR (for eyes tracking), in night vision and surveillance.
With TV displays, the quantum dot has proven to be a more efficient and energy-saving display method. Photoluminescent quantum dots are highly desirable in the next wave of displays. Utilizing QDs in the display technology such as LCD or OLED improves the colors gamut, enhances brightness, save energy (making them a more economical option) and cost less to manufacture. Quantum dot display potential is not a recent discovery. Brands like Samsung launched their QLED display TVs back in 2015, setting the stage for other companies to follow. A quantum dots display can come via virtually any method (prints, rolls, or folds) and size you can think of, because it can be deposited on any layer provided. Thus, it has a wide range of options with which it can be utilized in display.
Quantum dots also can be used as an active electroluminescent material in LED. QD LEDs is an emerging technology that promises to enhance current displays with higher brightness, durability and color purity. It will be an ultimate solution for flexible and curved displays in TVs, mobile and wearable devices, virtual and augmented reality glasses, automotive displays and signage.
Quantum dots have proven also very useful as energy saving, electricity producing elements in solar cells. Quantum Dots can be assembled into the solar cells by printing methods that comparable with traditional high temperature/vacuum deposition methods is relatively inexpensive, and it requires little energy in the manufacturing phase. While this process is not standard practice yet, it is in the works, and it looks promising.
Quantum dots applications in medicine include bio-labeling, bio-sensing, imaging, and targeting. In fact, when used in biomedical imaging, quantum dots can lead to early cancer detection, which as we know is crucial to survival. Quantum dots are preferred over conventional methods for imaging because of their photo-stability, high quality, size-tunable emission, and improved signal brightness. All of these properties are very important in order for the medicine to move upwards. Quantum dots can completely revolutionize medicine, making possible things that were only imagined a few years ago. Some concerns exist regarding the toxicity of the quantum dots when they are used for medicine. Please note that thorough, ongoing research is being done on this subject.
With the discovery of quantum dots, the world has achieved more feats than it previously thought possible. Even if we solely consider the field of medicine, these groundbreaking discoveries have successfully aided the growth and betterment of human life. The world is always changing; as technology keeps advancing, it constantly upgrades current innovations, and quantum dots have played a significant part in that process. Quantum dots have come a long way since their discovery, and we have them to thank for many of the benefits we enjoy today. The significant breakthroughs in quantum dot technology, have secured it a place in the world today, and we expect to see more of its impact in the future.
Useful links:
https://www.nanowerk.com/what_are_quantum_dots.php
https://medium.com/iete-sf-mec/quantum-dots-2cddb4f96e8c
https://en.wikipedia.org/wiki/Quantum_dot
https://link.springer.com/article/10.1007/s41061-016-0060-0
https://pubs.rsc.org/en/content/articlehtml/2010/cs/c0cs00055h
https://pubs.acs.org/doi/10.1021/acs.jpclett.7b01640
https://www.sciencedirect.com/topics/engineering/quantum-confinement-effect
https://onlinelibrary.wiley.com/doi/full/10.1002/anie.201708510
https://www.sciencedirect.com/topics/engineering/quantum-confinement-effect
https://www.sciencedirect.com/science/article/pii/S2090997714000212
