What are perovskite materials?
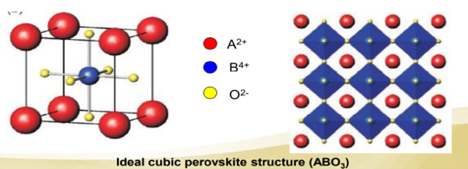
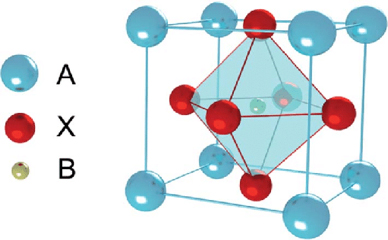
Perovskite is a calcium titanium oxide mineral, with the chemical formula CaTiO3. The terms “perovskite” and “perovskite structure” are often used as if they were interchangeable. In fact, true perovskite (the mineral) is made up of calcium, titanium, and oxygen in the form CaTiO3, whereas a perovskite structure is anything that has the generic form ABX3, and the same crystallographic structure as perovskite (the mineral). In a perovskite structure, ‘A’ and ‘B’ are two cations of very different sizes, and X is an anion that bonds to both. Perovskite is found in contact carbonate skarns at Magnet Cove, Arkansas, in altered blocks of limestone ejected from Mount Vesuvius, in chlorite and talc schists in the Urals and Switzerland, and as an accessory mineral in alkaline and mafic igneous rocks.
Where do perovskite materials come from?
Perovskite is a calcium titanium oxide mineral composed of calcium titanate (CaTiO3). The name “perovskite” is also applied to the class of compounds which have the same CaTiO3 (XIIA2+VIB4+X2−3) crystal structure, which is known as the perovskite structure. Many different cations can be embedded in this structure, which makes it possible to develop diverse engineered materials. Gustav Rose discovered perovskite in the Russian Ural Mountains in 1839, and named it after Russian mineralogist LevPerovski (1792–1856).
How are perovskite materials made?
Perovskite structures have the generic form ABX3, and any crystallographic structure in this form is assigned this name. True perovskite material is made in the form of CaTiO3 from oxygen, titanium, and calcium. Nevertheless, the word perovskite also refers to a type of ceramic oxides with the formula ABX. These compounds are known as alkaline metal halides perovskites, inorganic oxide perovskites, and organic metal halides perovskites. Bridgmanite is a silicate with the formula (Mg, Fe) SiO3, and is the most common mineral in Earth’s mantle. It adopts the perovskite structure under high pressures. Compared with SiO68− octahedral units, the SiO44− tetrahedral units in the dominant silica-bearing minerals become unstable as pressure rises. The second most abundant element is possibly the (Mg, Fe) Oxide-structured rock salt periclase, under the pressure and temperature conditions of the lower mantle.
How do perovskite materials relate to the quantum dot manufacturing industry?
Perovskite materials are the rising stars of the photovoltaic industry. They are cheap to manufacture, easy to make, and extremely effective. Better still, they are new to the scene, so much remains to be explored in terms of even more powerful solar cells. Perovskite materials are used in LED technology as well. Quantum dots (QDs) are a very useful class of semiconductor material with fascinating light-emitting properties, including the ability to adapt to whatever wavelength light is emitted to both LEDs and solar cells.
A new class of quantum dot based on perovskite materials is currently under development. Perovskite quantum dots are nanocrystal semiconductors. They are more resistant to defects than metal chalcogenide quantum dots, and they have excellent quantum yields of photoluminescence and high colour purity that have already equalled or surpassed metal chalcogenide QDs. Such properties are highly desirable for electronic and optoelectronic applications, meaning that perovskite quantum dots have tremendous potential for applications in the real world, including LED displays and solar cells with quantum dots.
Perovskite materials are receiving significant attention from the research community because of their outstanding photovoltaic performance. It has recently been shown that reducing the dimensions of a perovskite crystal structure to just a few nanometres creates quantum dots with very high photoluminescence quantum yields and excellent colour purity.
These quantum dots are highly robust, since they do not require any surface passivation* to retain their high PLQY (the photoluminescence quantum yield, or PLQY, of a molecule or material is defined as the number of photons emitted as a fraction of the number of photons absorbed). In the case of defect and trap locations, their energies are positioned beyond the bandgap, either in the conduction or valence bands. These perovskite material nanocrystals are easy to synthesise in a colloidal suspension, and are also easily incorporated into optoelectronic devices using readily available processing techniques. This means that they have a lot of potential for use in future technologies.

Perovskite Quantum Dot Applications
Perovskite quantum dots are less well researched than other types of quantum dots. However, perovskite quantum dots have thus far proven to be extremely effective in optoelectronics and nanotechnology, in a variety of different applications. For instance, perovskite quantum dots were used to build solar cells with power conversion efficiencies that surpassed those of comparable devices based on more traditional nanocrystal semiconductor materials.
Potential applications for perovskite quantum dots include:
- X-Ray Image Sensors
- UV Image Sensors
- Light Emitting Diodes
- LCD Displays
- Solar Cells
- Single Photon Sources
- Lasers
- Photodetectors
- Quantum Computing
- Cell imaging
- Cancer mapping


